Our animals consume more than 13 billion pounds of feed each year. Ensuring the quality, consistency and safety of our feed supply is critically important. Our companywide feed and food safety programs follow the requirements of the FDA Food Safety Modernization Act (FSMA). Our FSMA-based safety plan is customized for each feed mill and bakery meal operation and maintained with ongoing employee training. Precise formulas of corn, soybean meal, wheat and minerals and vitamins, when fed in the proper amount at the right time, allow our animals to grow and gain lean muscle. Our animal nutrition experts routinely study ways to improve the efficient use of our animal feed, analyzing raw ingredients and finished feeds for nutritional content and quality. They also work with our research and technology group to evaluate the impact of novel feed concepts and additives on animal performance.
At Smithfield Foods, our Animal Care Management System provides a comprehensive approach to animal care on all our farms. It includes employee training and audits to make certain that our animal care policies are followed at all times and that any issues of noncompliance are swiftly corrected. We created this system for our farms nearly two decades ago in consultation with two of the world’s foremost experts in animal behavior and handling.
We established a corporate-level Animal Care Committee in 2002 to ensure our animal policies are properly implemented. The committee, which consists of Smithfield employees with animal care responsibilities at our farms and processing facilities, reviews our policies at least once annually and communicates the importance of our program throughout the organization.
To ensure the safety and well-being of all our animals, we created an Animal Handling and Welfare Quality Management Plan. Used at all Smithfield processing facilities in the United States, this plan reinforces our Animal Care Policy; includes our animal handling program and supplier expectations; and identifies required personnel, training, auditing and adherence to regulations.
Our Animal Care Management System provides a comprehensive approach to animal care on all our farms. It includes employee training and audits to make certain that our animal care policies are followed at all times and that any issues of noncompliance are swiftly corrected.
All hog farms must adhere to the guidelines of the National Pork Board (NPB) Pork Quality Assurance® Plus (PQA® Plus) program. PQA® Plus provides guidelines for proper care of animals to ensure optimal health and well-being. It includes on-farm assessments and third-party verification that proper care is being implemented. In addition, the Common Swine Industry Audit (CSIA) verifies that we are following industry standards and that our farms are compliant with our Animal Care Policy.
All drivers who transport our animals, including contract and independent supplier drivers, must be trained and certified under the NPB’s Transport Quality Assurance® (TQA®) program. TQA® provides education and guidelines for transporters, producers and animal handlers on all aspects of hog handling and transportation.
Animals are treated with respect at processing facilities, just as they are when growing at farms. Each facility uses a systemic approach to animal care that includes the Smithfield Animal Handling and Welfare Quality Management Plan, a comprehensive training program, internal audits and third-party audits.
Animal Care Requirements
To implement our Animal Care Policy and make sure that animals are properly cared for, we rely on a comprehensive system of policies and procedures as well as internal and third-party auditing platforms.
Some of the requirements are specific to our hog farms; others are expressly for processing plants. We constantly assess these tools to be sure we are following current science that promotes the most humane treatment of animals.
On U.S. Farms
All our farms in the United States are 100% compliant with the NPB PQA® Plus program, which serves as the basis for the CSIA. To learn more about third-party certifications and audits, please visit the Industry Certifications and Verifications section.
Each farm’s compliance with the PQA® Plus standard is reviewed every three years. To ensure we remain compliant between reviews, our trained internal auditors conduct annual animal care audits, aligned with the CSIA, on company-owned farms.
We consistently strive for an audit score of “excellent” (97% or above) across all our farm regions, including our genetics research facilities. All farms are audited annually; in some cases, farms may be audited as groups, resulting in a lower number.
External auditors, who are retained by Smithfield Foods, conduct random, unannounced visits at company-owned farms to perform the CSIA. External auditors evaluate “big-picture” issues, including whether our internal auditors assess performance consistently across locations. Any audit findings are reviewed by management. Sites that fail an external audit are reaudited within 30 days, must show that any nonconformance has been corrected and will also undergo an audit in the subsequent year.
At U.S. Facilities
Our facility management system follows the standards set in the U.S. Department of Agriculture’s (USDA’s) Process Verified Program (PVP) and monitors several key aspects of production, including traceability to farm of origin, PQA® Plus program adherence on farms and TQA® status of livestock haulers.
Our programs help ensure the animals that come to the facilities were raised where management systems address health, animal well-being and proper use of antibiotics.
A third-party company performs annual audits at all our fresh meat-processing facilities based on North American Meat Institute (NAMI) guidelines.
In addition to regulatory oversight and enforcement by the USDA Food Safety Inspection Services, which has representatives stationed inside each of our locations every day of operations, all facilities are audited on a pass-fail basis by Smithfield employees at least once during each shift.
Our International Operations
Animal handling protocols at our locations in Poland and Romania include comprehensive document controls to ensure traceability, rigorous biosecurity protocols that meet all national and European Union (EU) regulations, proper hygiene measures and humane euthanasia.
Our international operations are regularly audited to ensure compliance with local and EU regulations. Government veterinarians regularly inspect our farms and facilities in both Poland and Romania. These external audits verify compliance with national animal care laws and biosecurity measures to reduce the risk of diseases. Additional random inspections take place throughout the year.
Trained internal auditors conduct audits of farming operations to verify compliance with animal care procedures, biosecurity and traceability, employee training programs and transportation systems. Nonconformance is addressed with swift corrective action, and we provide support and technical assistance to help each facility remain compliant.
Animal Care Policy Statement
Smithfield is committed to being an industry leader in animal care practices to ensure respectful and humane treatment of animals; to produce wholesome food products; and to analyze our operations and practices, including internal and independent third-party audits, to ensure continual improvement.
All operations involved with the production or processing of live animals are required to provide:
- Comprehensive written animal care programs to ensure animal well-being
- Shelter that is designed, maintained and operated to provide a physical environment that meets the animals’ needs
- Access to adequate water and high-quality feed to meet animal nutrition requirements (production facilities) and in accordance with the Humane Methods of Slaughter Act 1978 (processing facilities)
- Humane treatment of animals that ensures their well-being and meets or exceeds all applicable legal and regulatory requirements, including the Humane Methods of Slaughter Act of 1978 and all applicable NAMI Animal Handling Guidelines (processing facilities)
- Identification and appropriate treatment of animals in need of care
- Timely use of humane methods to euthanize sick or injured animals not responding to care and treatment
Adherence to the principles of this policy is a responsibility and requirement of those who interact with animals that are owned or processed by Smithfield. Willful neglect or abuse of animals will not be tolerated and will result in immediate termination. Offenders may also be subject to criminal prosecution under applicable laws.
Smithfield Foods’ genetics research center has one primary aim: making a better pig.
This is done through the genetics equivalent of matchmaking. Researchers select animals from generation to generation, pairing them up to create the ideal descendants for that perfect rack of ribs or the tastiest ham. We determine which animals will make the best parents to produce the best offspring that will result in the tastiest meat. The meat we produce today isn’t something that happened by accident. It’s the result of years of genetics research and effort.
In facilities in North Carolina and Texas, more than 200 technicians, genetic researchers and veterinarians look for new ways to improve the genetic traits of the animals, focusing on everything from a sow’s nursing skills to a piglet’s feeding abilities to the characteristics that result in the greatest flavor with the perfect amount of fat and marbling.
We also select for specific genetic traits that will keep our animals comfortable and healthy. Our teams collaborate with other researchers across the United States and internationally, focusing on a host of features, including a pig’s ability to efficiently gain weight.
Using a variety of statistical tools, we collect hundreds of animal traits and analyze them to determine which are the best ones for the next generation going forward. We manage a range of objectives, from growth performance to tenderness to yield. And, of course, flavor. This type of work takes time—once genetic improvements are identified, it can take as many as five years for those changes to be represented in our food products.
We firmly believe that Smithfield hogs offer a superior taste and eating experience. Our hogs raised today are a combination of three heritage breeds: 100% Durocs on the male side and a 50–50 cross between Landrace and Large White hogs on the female side. Although the three lines are common worldwide, the hogs we have bred are unique within our industry. Our genetics program itself is also unique. Other meat companies in the United States use third parties to develop their breeding programs. Thanks to our vertically integrated research, we can literally trace the genetic lines across our entire pork chain, from breeding to farms to the final product.
In addition, our hogs today require fewer resources to raise, thanks to a targeted effort by our geneticists to identify characteristics that enable us to raise animals more efficiently. For example, some humans gain weight more easily than others, the result of the genes they inherited. We want to produce hogs that gain weight more easily. That’s because hogs that convert calories more efficiently require fewer resources (and fewer days) to grow from infancy to market weight.
The swine genome became available in 2009 and is the primary tool we use in our work today. From that data, we were able to refine our abilities to capture DNA information and use it to screen animals that will parent the next generation. It typically takes three to four years before we see the selections we have made show up in the animals on our farms.
We also keep in mind biodiversity, so we can ensure a diverse population of hogs. In other words, we breed across familial lines.
Outside of their labs, our geneticists spend quite a bit of time educating Smithfield employees about what they do. The program that describes their work, “The Smithfield Experience,” has trained thousands of Smithfield employees over the years, giving them insight into our breeding program and why it is so successful.
Gene Editing
At Smithfield Foods, it’s important to note that our robust genetic program does not currently include gene editing. We do not add or manipulate genes.
Rather, we employ the science of genomics, which includes calculating thousands of genetic data points to accurately predict characteristics for the next generations of hogs. The science involved in gene editing is still evolving. The company’s focus remains on the development and improvement of its products through careful selective breeding and genetic research. Smithfield will continue to monitor and study scientific research on gene-editing technology for potential future opportunities.
Genetically Modified Organisms (GMOs)
Meat and poultry livestock are not GMOs. However, most of our animals are fed grains (including corn and soybeans) that may have used GMO technologies.
All GMO crops have been evaluated by a host of regulatory agencies, including the U.S. Food and Drug Administration (FDA) and the U.S. Environmental Protection Agency (EPA), as well as many scientific organizations, and have been found to be safe for people and animals to eat. There is no evidence that animals are affected by eating grain from plants with genetically modified characteristics.
At Smithfield, we monitor the dialogue about GMOs and stay current with scientific research. We do not require any of our suppliers to be non-GMO.
Cloning
We do not produce protein products from cloned animals and have no plans to do so in the future. Although the FDA has concluded that protein products from cloned animals are safe for human consumption, the science involved in cloning animals is evolving. We will continue to monitor further scientific research on this technology.
We maintain our focus on the development and improvement of our protein products through careful selective breeding and genetic research.
Smithfield Foods is committed to being an industry leader in animal care practices that ensure respectful and humane treatment of animals. Housing systems for all animals are designed, maintained and operated to provide a physical environment that meets the animals’ needs and complies with all applicable legal and regulatory requirements.
Group Housing for Female Pigs
Smithfield was the first company in its industry to commit to group housing systems for sows in its company-owned operations in 2007 and invested $360 million to convert its farms accordingly. Today, all Smithfield company-owned farms employ group housing systems. Smithfield has encouraged its suppliers to invest in and implement group housing systems and provides guidance to aid with the conversion process upon request.
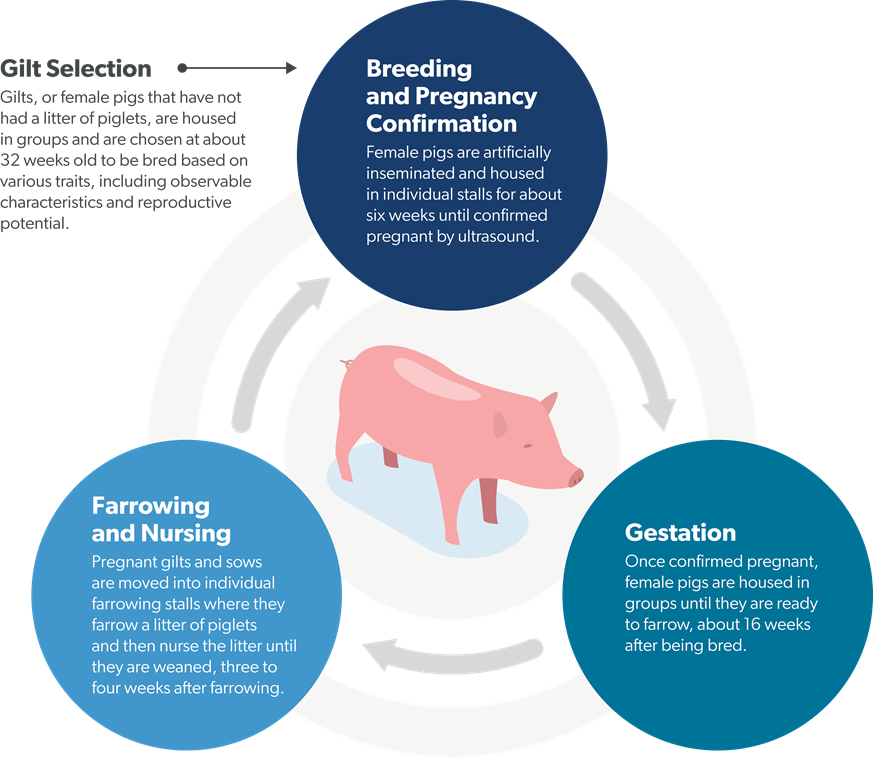
Smithfield’s company-owned sow housing systems have three components: individual stalls, group pens (“free access” or “small group” pens) and farrowing stalls for giving birth. Sows stay in individual stalls for 35-42 days after they are bred to allow for individual care and to minimize fighting between animals during this early, high-risk stage of the pregnancy. Once confirmed pregnant, sows are moved to a group pen where they stay for 68-75 days.
There are two types of group pens on Smithfield’s farms:
• Free access group housing allows the sow to choose between a common area for lounging and exercise - or individual stalls that they can come and go from as they please.
• Small group housing provides a common area for lounging and exercise with access to shoulder stalls for feeding. Close to their due date, sows are moved into individual farrowing stalls for approximately 27 days.
Watch a video about Smithfield’s group-housing system
here.
As one of the world’s largest pork producers, biosecurity, or procedures to prevent the spread of disease to our farms, is a critical element of our program to safeguard the health of our animals.
Strong biosecurity on our farms, and throughout the industry, is not only vital to our business, it also supports our efforts to help feed a growing world population, provide jobs in our communities and sustain other businesses in our supply chain such as corn and soybean farming.
Smithfield Foods’ standard operating procedure covers the animal-production process at individual farms as well as the movement of vehicles, animals, personnel and equipment between farms. This policy is strictly enforced at all our company-owned and contract grower farms. To stay up to date and ensure our program remains strong and informed by current science, we monitor emerging and ongoing animal disease threats around the world and collaborate with relevant regulatory agencies and other industry experts.
Our biosecurity procedures focus on preventing contaminants from being brought onto farms; for example, employees and visitors must “shower in” and change into clean clothing before entering all sow farms and must also “shower out” prior to leaving. In addition, equipment and supplies delivered to sow farms, as well as vehicles, must be disinfected prior to being allowed inside the farm complex.
There are times on farms when employees must humanely euthanize pigs following injuries or illnesses. Employees are trained by our veterinarians in accordance with the recommendations of experts, including the American Association of Swine Veterinarians (AASV) and the NPB.
In recent years, we have been reviewing our operating procedures around euthanasia to ensure that we are using the most appropriate methods, based on the size and weight of the animals involved.
We have invested in research to understand which techniques cause the least pain and stress to the animals and to their handlers. For pigs weighing less than 65 pounds, we use either carbon dioxide (CO2), which causes painless loss of consciousness and death, or a device called a nonpenetrating captive bolt gun, which administers a controlled blow to the head without breaking the skin, instantaneously rendering the animal insensible and causing a quick death. For pigs larger than 65 pounds, we use a penetrating captive bolt gun that fires a retractable metal bolt into the brain, resulting in insensibility and death.
According to the AASV, humane methods will achieve the following:
- Minimize pain and distress to the pig during administration.
- Cause rapid loss of consciousness.
- Result in death quickly and consistently.
Humane Slaughter Methods
Smithfield Foods has led the U.S. pork industry in installing equipment to anesthetize pigs using CO2. Our facilities use the Butina® CO2 Backloader anesthetizer system, which allows pigs to move slowly, in small groups, minimizing stress for the animals and their handlers. CO2 anesthetizing is very effective and produces higher-quality meat than the older, single-file electrical stunning systems.
Our international pork operations also utilize CO2 anesthetizing, while poultry operations use both CO2 and electrical water-stunning methods. While we primarily use CO2 anesthetizing, we do use electrical stunning at one recently acquired sow harvest facility in Iowa. This method is recommended and published by the NAMI as an effective method to comply with federal humane slaughter regulations as well as promoting animal welfare and meat quality.
African Swine Fever (AFS)
A highly contagious viral disease that is nearly always fatal to pigs and for which there is currently no treatment or vaccine
Barrow
A male pig that has been castrated
Boar
An intact male that has not been castrated
Boar Taint
An offensive odor or taste that can be evident during the cooking or eating of pork and comes from noncastrated male pigs once they reach puberty
Clean Labels
An effort to make product labeling shorter and easier to understand for the consumer; the Ingredient Glossary provides more information about our ingredients
Contract Grower
Private landowners and independent farmers who are paid under agreements that typically run for multiple years; Smithfield Hog Production assumes the market risks and owns the hogs, while the growers are protected from market fluctuations and receive a predictable income stream
Independent Supplier
A significant source of hogs for us and are important to our business, as they sell their animals directly to our processing facilities in the United States
Gilt
A female pig that has not had a litter of piglets
Market Pig
A pig that has reached the target market weight (about 285 pounds) and is ready to be harvested
Ractopamine
A feed supplement to help produce leaner meat more efficiently and is a safe and effective FDA-approved supplement
Sows
A female pig that has had a litter of piglets; also known as a mother pig
Sow Farms
Where gilts and sows are bred, gestate and farrow litters; piglets grow to 15 pounds on this farm
Traceability
The ability to trace our hogs back to the farm of origin
Wean-to-Finish Farms
Where piglets are moved to grow to market weight after sow farms
Ractopamine is a safe and effective FDA-approved feed supplement that has been used by hog and beef producers for many years to produce leaner meat more efficiently. The supplement, which is widely used in the United States, can be part of a healthy, balanced diet for growing hogs.
However, a number of nations ban the use of ractopamine. China, Russia and the EU countries, for example, require third-party verification that pigs are not fed ractopamine. To meet this demand, we have removed ractopamine from feed for all animals supplied to our processing facilities. Several Smithfield plants now produce meat from pigs that have never received ractopamine. We still have facilities that receive pigs from other suppliers that use this product. We also have initiatives with our producers to let them participate in our “never fed ractopamine” program if that fits with their production capabilities.